Redox Biology 101
|
Reduction-oxidation (redox) reactions involve the transfer of electrons between chemical species. These reactions play a role in many common processes, such as cellular respiration and metal corrosion, and can also result in the formation of free radicals, which in high levels cause significant cellular damage. As a result, redox biology is an important area of research. Here you’ll find a quick overview of redox biology and its role in free radical formation as well as protein-cysteine oxidation. Redox reactions involve simultaneous oxidation and reduction reactions. Oxidation is the loss of electrons, or an increase in oxidation state, while reduction is the gain of electrons, or a decrease in oxidation state. The redox potential (E) of a chemical species refers to its tendency to acquire electrons and therefore be reduced. The transfer of electrons can result in the creation of free radicals, or chemical species with unpaired electrons in their outer orbits. When the free radicals contain oxygen molecules, they are known as reactive oxygen species (ROS). Three different ROS byproducts can be generated as oxygen is reduced to water. If one electron is gained, superoxide anion (O2–) is formed. If a second electron is gained, hydrogen peroxide (H2O2) is formed. And if a third electron is gained, the result is a hydroxyl radical (•OH), the most reactive of the three and therefore the most damaging to cells. The gaining of a fourth electron creates water. The Impact of Free Radicals The generation of free radicals due to the partial reduction of oxygen can occur under normal physiological conditions during oxidative phosphorylation, the formation of cellular ATP. It can also result from the following sources:
Oxidative stress occurs when your body cannot counteract or neutralize the generation of these free radicals, resulting in high ROS levels. This causes various types of cellular damage. For example, oxidative stress triggers lipid peroxidation, which destabilizes cellular membranes. It also results in the oxidation of amino acids in proteins, which can lead to non-functional enzymes, as well as oxidation of DNA. These DNA mutations can then lead to cancer. To prevent this damage from occurring, the body has several forms of defense:
|
|
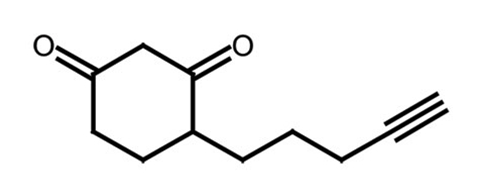
Protein Cysteine Oxidation In protein cysteine oxidation, susceptible cysteine residues within a protein undergo oxidative modification by mild oxidizing agents such as hydrogen peroxide, alkyl hydroperoxides and peroxynitrite. A natural cysteine contains a thiol (SH) group, which can be oxidized to sulfenic acid (SOH). Sulfenic acid can be oxidized to sulfinic acid (SOOH), which in turn can be oxidized to sulfonic acid (SOOOH). These modifications are of considerable biological interest as important players in redox catalysis and regulation, as well as sensors of oxidative stress. However, they have been notoriously difficult to identify due to their high reactivity, particularly outside their native protein environments. |
 |
|
To address that challenge, several Kerafast providing investigators have made protein cysteine oxidation probes available to the global research community. The probes are amenable to in vitro and in vivo cysteine sulfenic acid detection. Many of the available probes detect sulfenic acid; a dimedone moiety is commonly used to recognize the SOH group, though several probes are not dimedone-based. Available sulfenic acid probes include:
A sulfinic acid probe is available as well. Sulfinic acid formation has become a hotter topic of research as increasing evidence indicates that cysteine is oxidized to sulfinic acid in cells to a greater extent, and with more control, than originally thought. Scientists had long considered sulfinic acid formation an irreversible state of oxidation, but the discovery of sulfiredoxin demonstrated that cysteine sulfinic acid can be reversed, pointing to a vast array of potential implications for redox biology. Do you work in this area of research? Check out our other available reagents for redox biology research, including redox potential-tuned azurin proteins with redox potentials spanning the entire physiological range for use as tunable and water soluble redox agents, as well as antibodies for advanced glycation end products (AGE) that are found on proteins and lipids as a result of oxidative stress and chemical glycation. |