Photoreactive HDAC Inhibitors: A New Way to Form A Stable Relationship with HDACs
Marcos Salazar
March 10, 2014
The deregulation of histone deacetylases (HDACs) has the potential to instigate various cancers and neurodegenerative diseases. Dr. Pavel A. Petukhov has cleverly designed HDAC inhibitors containing a photoreactive element that selective form a stable bond with HDACs and inhibit their detrimental effects.
Histone deacetylases (HDACs) have managed to maintain a steady state of popularity due to extensive research that has revealed them to be hot drug targets for multiple therapeutic areas. The most notable areas of research that have benefited from targeting HDAC activity are those related to various types of cancers and neurodegenerative diseases. So why are HDACs so important for disease initiation and progression?
HDACs are a family of enzymes that are vastly responsible for epigenetic mechanisms inducing chromatin remodeling and transcription regulation. Through this strict regulation, HDACs are able to control various cell processes, such as cell growth, differentiation, proliferation, cell cycle progression, DNA repair mechanisms, and apoptosis. In mammals, 18 distinct HDACs have been found that vary in functionality, but in essence, deacetylate lysine residues from histones. The deacetylation process increases the ionic interactions between positively charged histones and negatively charged DNA. The strong interactions lead to a more compact chromatin structure and repression of various genes.
What’s so intriguing about HDACs is the identity of the repressed genes: most of the transcriptionally silent targeted genes encode for tumor suppressors, cell cycle inhibitors, differentiation factors, and apoptosis-inducing proteins. Various cancers, such as carcinomas and sarcomas, have even been shown to overexpress certain HDACs. As is evident, all these events contribute to a perfect environment for cancerous cells, therefore revealing HDACs as quite the supporters of disease development and progression.Extensive research has pointed to HDAC inhibitors as a promising strategy and plausible treatment to fight off cancer. HDAC inhibitors function to regulate HDAC activity, inducing an increase in acetylation and subsequently promoting expression of genes responsible for controlling cancerous growth. HDAC inhibitors are able to cause cell-cycle arrest and also inhibit DNA repair mechanisms, sensitizing cancer cells to chemotherapy and radiotherapy treatments.
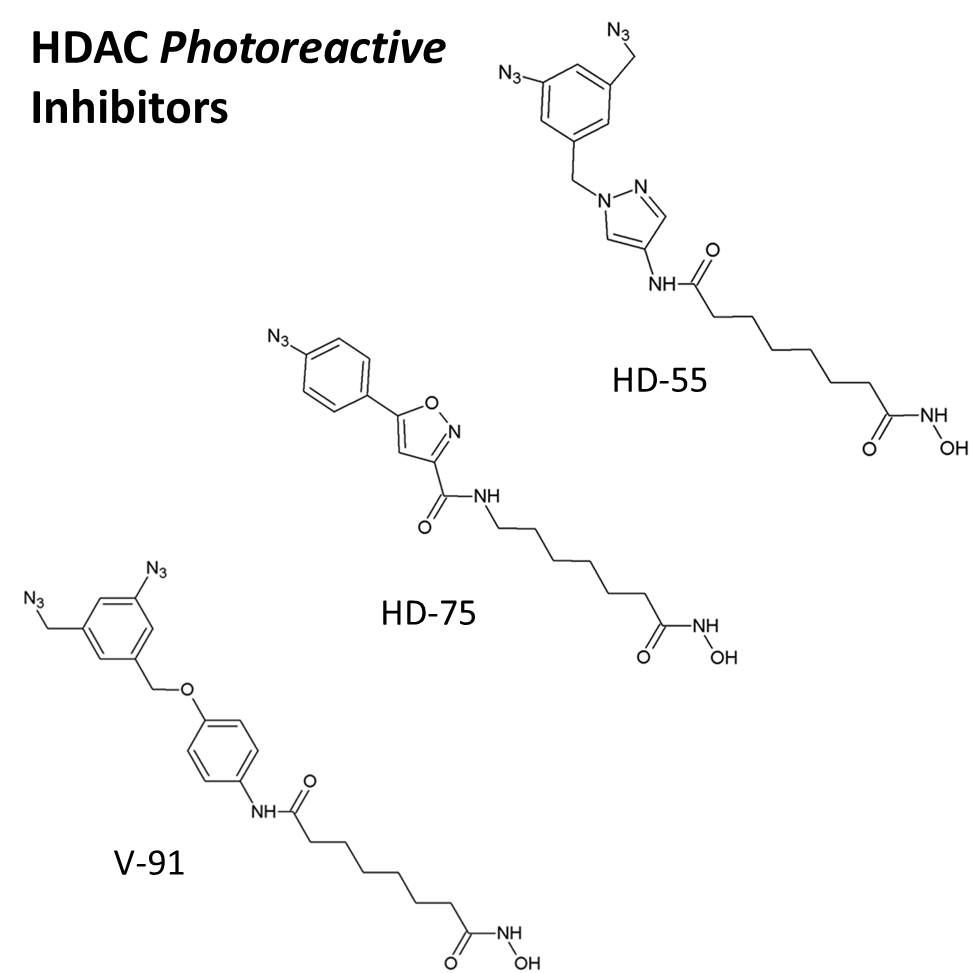
Pavel A. Petukhov, an investigator at the University of Illinois at Chicago, is one of the many researchers to take on the challenge of defining the importance of HDAC inhibition. To address this task, Dr. Petukhov and his research team designed highly potent and selective probes for HDAC3 and HDAC8. What makes his probes so unique is the fact that he’s managed to select for probes with inhibitory effects against HDACs that also contain a photoreactive element to them.
The photoreactive probes, or HDAC inhibitors, have been created through a photoaffinity labeling method, incorporating an aryl azide group that gives these inhibitors their wow factor. Aryl azide groups are a staple in photoaffinity labeling experiments, as they are highly reactive, universal, and simple to implement in many experiments. Upon activation of the photoreactive group of the inhibitor by UV exposure, a covalent bond is formed between HDAC and the inhibitor itself. The positioning of the photoreactive aryl azide group, as well as the flexibility of the inhibitor itself, allows for the inhibitor to adopt a conformation complementary to the HDAC target active site.
Dr. Petukhov’s photoreactive HDAC inhibitors have been shown to suppress cell proliferation in several cancer lines, as well as offer protection against chemically-induced apoptosis in neuronal cells. HDAC inhibitor HD-75 is selective for HDAC3 and has proven useful for mitigating the cancerous behavior of gastric, prostate, and colorectal origin. HDAC inhibitors HD-55 and V-91 are selective for HDAC8 and are more effective against neuroblastomas and leukemias. Of notable interest is that HD-55 is actually more selective and exhibits a significantly lower half maximal inhibitory concentration (IC50) than suberoylanilide hydroxamic acid (SAHA), a currently FDA-approved HDAC inhibitor for T-cell lymphoma.
These photoreactive HDAC inhibitors are promising new research tools that will surely make an impact on studies of diseases influenced by HDAC activity. They are useful for molecular modeling, drug design applications, rapid prediction of protein-ligand interactions, identification of protein candidates for drug targeting, and mapping of target binding sites. On behalf of Dr. Petukhov and our team here at KeraFAST, we welcome you to learn more about these HDAC inhibitors and see how they can be incorporated into your own research.
Learn more about Dr. Petukhov’s Photoreactive HDAC Inhibitors:
HDAC8-Selective Photoreactive Inhibitor HD-55
HDAC3-Selective Photoreactive Inhibitor HD-75
HDAC Photoreactive Inhibitor V-91
Tags:
histone deacetylases, HDAC, HDAC3, HDAC8, HDAC inhibitor, HDACi, HDIs, photoreactive, photoaffinity labeling, epigenetic, transcription silencing
References:
- He, B., et al. Binding Ensemble Profiling with Photoaffinity Labeling (BEProFL) Approach: Mapping the Binding Poses of HDAC8 Inhibitors.Journal of Medicinal Chemistry. 52(22): 7003 – 7013. 2009.
- Neelarapu, R., et al. Design, Synthesis, Docking, and Biological Evaluation of Novel Diazide-Containing Isoxazole- and Pyrazole-Based Histone Deacetylase Probes. Journal of Medicinal Chemistry. 54: 4350 – 4364. 2011.
- Dokmanovic, et. al. Histone Deacetylase Inhibitors: Overview and Perspectives. Molecular Cancer Research. 5 (10): 981-989.
- Glozak, M.A. and Seto, E. Histone deacetylases and Cancer. Oncogene. 26: 5420-5432.



