Hydrodabcyl: a superior hydrophilic alternative to the dark fluorescence quencher dabcyl
Dark fluorescence quenchers are non-fluorescent dyes that can modulate the fluorescence signal of an appropriate fluorophore donor in a distance-dependent manner. Dark quenchers are often used in dual-label probes, which combine a reporter dye with a quencher moiety. Typically, these probes may exist in two conformations differing in their fluorescence properties: a ‘closed’ form in which the reporter and the quencher are in close proximity and an ‘open’ form in which these groups are spatially separated. A very common application of such dual-labelled probes are fluorogenic protease substrates, like glutathione.

One the projects of Bombarda Lab at the University of Bayreuth (Germany) consists in the investigation of the mechanism of the cleavage reaction catalysed by a cys-protease. The substrate of our enzyme is the tripeptide glutathione. Our strategy included the labelling of the natural substrate with a suitable fluorophore/quencher pair. As a dark quencher, we chose the very popular dabcyl. Unfortunately, the first assays indicated that the product is not released preventing enzymatic turnover. This is inconvenient and was attributed to the increased hydrophobicity of the substrate due to the presence of dabcyl, which is a hydrophobic azobenzene derivative. In fact, stock solutions of dabcyl (1) need to be prepared in DMSO. Although dabcyl (1) is one of the most popular acceptors for developing FRET-based probes, its insolubility in water severely limits its use in biological systems where the natural solvent is water. Even when this hydrophobicity can be partly compensated by the hydrophilicity of the substrate to which dabcyl (1) is linked (e.g., long DNA segments or peptide chains), it may cause a real problem in the case of comparatively small substrates, like glutathione.
This issue prompted us to develop a new dark quencher which retains the advantageous spectroscopic properties of dabcyl while being soluble in aqueous solutions. After brain storming with my co-workers, we came up with hydrodabcyl (2). US Patent 9815774-B1, EU Patent EP3031797-A1)
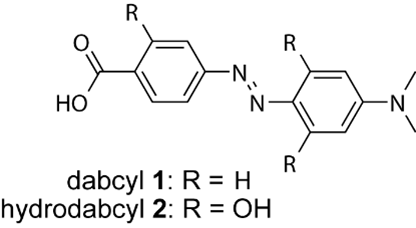
Technically, hydrodabcyl (2) (4-(2′,6′-dihydroxy-4′-dimethylaminophenylazo)-2-hydroxybenzoic acid, is a trishydroxy derivative of the parent dabcyl (1). The attachment of hydroxy groups to the dabcyl core confers water solubility without adding electric charges as would have the addition of ionic solubilizers such as sulfonate groups. Consequently, hydrodabcyl (2) will not significantly modify the electrostatic profile of the molecule to which it is linked and thus the binding properties of the labelled molecule are not expected to be altered much. This aspect has important implications for investigations of biological systems in which molecular interactions are frequently driven by electrostatics (e.g. enzymatic reactions). (Anal. Chem. 2017, 89, 11893−11897, DOI: 10.1021/acs.analchem.7b03488)
We challenged hydrodabcyl (2) by testing it with a dipeptide, which is the minimal substrate for a protease reaction. The dipeptide Ser-Phe was chosen because it is a preferential cleavage site of the P1’ protease thermolysin. Furthermore, the same dipeptide labeled with EDANS and dabcyl (1) had already been tested with several proteases, therefore allowing a comparison of dabcyl (1) and hydrodabcyl (2) during a typical biochemical application. In all the tests hydrodabcyl (2) proved to be superior to dabcyl (1).
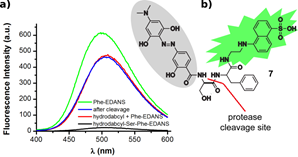
a) Fluorescence spectra of 20 μM hydrodabcyl-Ser-Phe-EDANS alone (black) and after 5 h incubation with thermolysin (blue). For comparison, the fluorescence spectra of 20 μM Phe-EDANS alone (green) and an equimolar amount of hydrodabcyl (2) + Phe-EDANS (red).
b) Chemical structure of hydrodabcyl-Ser-Phe- EDANS.
Credit: Analytical Chemistry
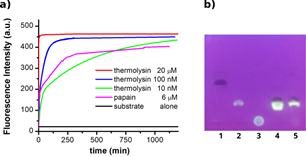
a) Time traces of the hydrolysis of 20 μM of hydrodabcyl-Ser-Phe-EDANS (7) at 37 °C, (λEx= 336 nm, λEm=515 nm) by thermolysin (different concentrations) and papain (6 μM).
b) Products of hydrodabcyl-Ser-Phe-EDANS after (7) proteolytic cleavage indicated by TLC: hydrodabcyl-Ser-Phe-EDANS (7) (lane 1, Rf = 0.5), Phe-EDANS (lane 2, Rf = 0.3), EDANS (lane 3, Rf = 0 – 0.1), reaction mixture after proteolytic cleavage with thermolysin (lane 4, bright spot
Rf = 0.3 and dark spot Rf = 0.33) and with papain (lane 5, bright spot Rf = 0.3 and dark spot Rf = 0.33).
The dark quencher, hydrodabcyl, (2) can be conveniently activated for amide bond formation in form of its N-succinimidyl ester, (3) which is stable in the solid state and may be stored for months in the fridge or as DMSO solution at room temperature. Hydrodabcyl N-succinimidyl ester (3) reacts selectively and swiftly at room temperature with primary and secondary amines in good to quantitative yields. This selective amide bond formation takes place also in the presence of other protein-typical nucleophilic functional groups, such as hydroxyl, carboxyl, thiol, and the aromatic imino groups of imidazole and indole. Hydrodabcyl N-succinimidyl ester (3) can even be used in water. Furthermore, mono-Boc protection of hydrodabcyl (2) proceeds regioselectively affording a derivative with properties convenient for organic synthesis of other labelled compounds (publication submitted).
Finally, hydrodabcyl (2) is a new dark fluorescence quencher with an optimal solubility-stability-absorption profile. Its small dimension, the absence of charged groups, and its absorption range make hydrodabcyl (2) the dark quencher of choice in tandem with many commercially available fluorescence donors. Hydrodabcyl (2) overcomes the problem of insolubility in aqueous media, doing away with the need of organic co-solvents, and it lends itself ideally to high-throughput enzymatic tests. Thus, hydrodabcyl (2) represents a vastly improved and superior alternative to the very popular dabcyl (1) in the design of fluorogenic probes.


