Improving Zinc Ion Quantitation with Unique Biosensor Proteins and Buffers
In recent decades, an increasing number of research studies have centered on zinc. While scientists have long known zinc is a required nutrient for human health, the discovery of zinc transcription factors and zinc transporters with clinical applications led to an increased interest in the element. As a result, new tools and reagents have been developed to facilitate research into zinc biology.
In particular, zinc researchers have needed sensitive, selective and fast-acting tools to measure and image zinc ions, as well as buffers to calibrate those sensors. In a biological system, many cations are present in much higher quantities than zinc, such as calcium or magnesium. A challenge has been to develop zinc sensors with the ability to accurately track fluctuations in such small concentrations of zinc.

Richard Thompson, PhD, has been working for many years with collaborators across the zinc research field to address these challenges, resulting in the development of unique zinc biosensors and buffers. He founded the company Pokegama Technologies to improve access to these zinc reagents, and recently partnered with our team at Kerafast to facilitate the reagents’ distribution to scientists around the world. We sat down to chat with Dr. Thompson about the reagents, their development and applications, and what the future holds for his company. Read on below for our key takeaways from the interview.
1. Please describe the development of your zinc reagent collection. What research problems do the reagents address, and what advantages do they offer compared to other commercially available zinc biosensors and buffers?
The development of these reagents started years ago, a result of the US Navy’s interest in developing sensors for pollution control. Ships are covered in metal-containing paint, so the navy wanted to assess the potential for any metal pollution such as zinc occurring as a result of their ships. The navy connected me with two outstanding scientists in the field – Professors Carol Fierke, now at Brandeis University, and David Christianson, now at University of Pennsylvania – and the three of us set about developing better metal ion sensors, particularly for zinc.
Our sensors are based on the enzyme carbonic anhydrase. It was known since the 1960s that the zinc-bound form of carbonic anhydrase was the active form and could be made to fluoresce. Our team modified the recognition chemistry by changing the structure of the protein, and also the fluorescence reporting system. As a result, we improved carbonic anhydrase’s affinity for zinc (or how tightly it binds zinc ions), as well as its selectivity and kinetics.
Other available zinc sensors use small molecules, where typically a zinc binder is covalently attached to an absorbing or fluorescing moiety. Our Fierke-Thompson sensors are the only ones based on carbonic anhydrase. They are very sensitive, able to quantitate zinc ions down to the picomolar level. They are very selective, able to measure zinc in complex systems such as cytoplasm or sea water where potentially interfering metal ions are present at billion-fold higher concentrations. In addition, they are extremely fast-acting, allowing researchers to measure rapid fluctuations in zinc concentration. The sensors we currently offer are excitation ratiometric sensors, enabling accurate and reproducible calibration while minimizing most artifacts.
2. What are the key applications of zinc biology research?
Over the last 30 years, zinc has been found to be a player in all kinds of disease processes. In particular, zinc transporters have been linked to conditions such as cancer, diabetes, rare diseases, and memory formation in the brain. For example, an early genome-wide association study (GWAS) showed that one of the biggest genetic risk factors for Type II diabetes is a flaw in a zinc transporter.
In addition, the effects of having too much or too little zinc can be dramatic on the human body. It’s common for people to be zinc deficient. Iron deficiency, particularly in women, is well known but zinc is almost as big of a deal and much less appreciated.
3. Another emerging application for zinc research is the metal’s effect on viral propagation, in particular as related to the global COVID-19 pandemic. Could you please elaborate on zinc’s potential role in this process?
The reverse transcriptase in SARS-CoV-2, the virus that causes COVID-19, utilizes zinc as a cofactor; if you remove or interfere with its zinc, the enzyme is no longer active. Reverse transcriptase is an enzyme unique to viruses – humans don’t possess it – making it a good potential drug target. Also, a recent Spanish study found that COVID-19 patients who had low zinc typically also had worse outcomes from the disease. This is a small area of research compared to all the other COVID-19 drug development efforts; however, a group of zinc scientists are also pursuing this avenue of research.
4. As a life-long academic researcher, what has the process been like to create your own company? Why did you decide to partner with Kerafast to distribute your zinc reagents?
Researchers would ask us for our biosensors over the years, but it became clear to us that most people were continuing to use small molecule indicators for zinc research, which work fine but not for every application. If we wanted to make our protein-based sensor more widely available, we needed some sort of commercial availability. We also thought it would be useful to offer our zinc calibration buffers commercially. We had published recipes for making them, but it’s tedious to make them and most people choose to buy buffers if they can.
I knew about Kerafast from my research at the University of Maryland, and I knew it could enable a little company like mine to make the zinc reagents more widely available. I also wanted to enable orders from abroad. Kerafast is the expert with this; by working with them, I don’t have to put all the company infrastructure in place myself.
5. What does the future hold for Pokegama Technologies? Could you give us the inside scoop on any new products in the pipeline?
Our goal is to make it easy for people to do good experiments with zinc. We are planning to launch a variant of the biosensor protein that penetrates into cells using a TAT peptide, allowing researchers to look at zinc inside the cell or within specific organelles such as mitochondria. We are also planning to offer the DNA for biosensors, enabling scientists to transfect cells and measure zinc intracellularly. In addition, we are planning to offer a zinc additive that can be added to growth medium such as DMEM so that researchers can know the exact concentration of free, or bioavailable, zinc in their medium.
Pokegama Technologies is planning to have an exhibitor booth at the ASBMB/Experimental Biology meeting in Philadelphia this April; if you’re also attending, stop by to meet Dr. Thompson and learn more about the zinc reagents. In the meantime, please check out the reagents available via the Kerafast website.
We currently offer three fluorescent zinc biosensor proteins, used to measure and image zinc ions in cells and aqueous solutions in combination with a specialized cell-penetrating fluorescent aryl sulfonamide:
- WT-apoCA-tagRFP Biosensor Protein: highest zinc sensitivity, ideal for extracellular use
- E117A-apoCA-tagRFP Biosensor Protein: faster response to changes in zinc ion concentrations at low levels than WT
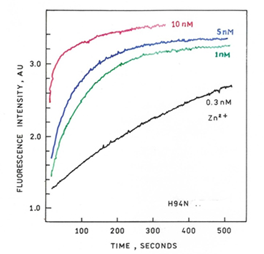
- H94N-apoCA-tagRFP Biosensor Protein: rapid response with sub-nanomolar sensitivity for measuring zinc changes
We also offer two MetalloBuffer™ Buffer Kits, which provide fixed, low zinc concentrations for calibrating sensors in a manner analogous to pH buffers, with zinc levels corrected for ionic strength and temperature effects:
- pZn 11-7 Buffer Kit: for zinc concentrations between 10 pM and 0.1 uM
- pZn 13-9 Buffer Kit: for zinc concentrations between 0.1 pM and 1.0 nM
Please contact us with any questions!


