Roll-out of a Malaria Vaccine Funded by Gavi
While the COVID-19 pandemic continues to dominate the world’s health-related news, other diseases have not slowed down their assault on the global population, especially in the poorest nations. Malaria remains an enduring health challenge, resulting in more than 400,000 deaths per year, including 260,000 children, according to the World Health Organization (WHO). However, there has been some promising news – in early December, the board of Gavi (formerly the Global Alliance for Vaccines and Immunisation) announced approval of funding for the roll-out of a malaria vaccine in sub-Saharan Africa.
About malaria
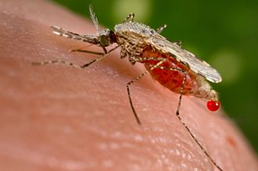
Malaria is transmitted to humans and other animals via the bite of a female mosquito carrying a single-celled organism of the Plasmodium genus. Symptoms include fever, tiredness, headache, coma and death. Widespread in tropical and subtropical regions, the disease is found particularly near the equator and much of sub-Saharan Africa, Asia and Latin America.
Preventative measures include mosquito nets, elimination of standing water (where mosquitos breed) and insecticides. Travelers to these regions often will take prescribed anti-malarial drugs; however, the impact of malaria on the local inhabitants of the region remains devastating both to public health and the economy.
Funding the vaccine roll-out
In a recent press release, Gavi shared the news about their Board’s decision to support a malaria vaccine program beginning in 2022, with $155.7 million for the introduction, procurement and delivery of the malaria vaccine to Gavi-eligible countries in sub-Saharan Africa.
Gavi, formed in 2000, is an international vaccine-focused organization that brings together the public and private sectors in an alliance with the “shared goal of saving lives and protecting people’s health by increasing equitable and sustainable use of vaccines”. The Gavi alliance includes partners such as UNICEF, The Bill & Melinda Gates Foundation, The World Bank, and The World Health Organization. They are complemented by developing and industrialized country governments, developing and industrialized pharmaceutical companies, civil organizations, and research centers.
Potential for the future
The first malaria vaccine, RTS,S, was shown to provide partial protection against malaria in young children and was piloted in Ghana, Kenya and Malawi. Three doses were given, followed by a booster dose demonstrating a decrease in clinical malaria, severe anemia, blood transfusions and hospitalization due to malaria. This vaccine holds a lot of promise to combat malaria and decrease its prevalence amongst the world’s poorest children.
Do you work in malaria research?
Kerafast offers a variety of malaria-related reagents that may be of interest for your research.
- Anopheles gambiae Alpha Amaylase [AgAmy1] Antibody from University of Georgia
- Anopheles gambiae Aminopeptidase [AgAPN2] Antibody from University of Georgia
- Adiopokinetic Hormone (AKH) Antibody from University of Georgia
- Ecdysteroid E/20E Antibodies and Antigens from University of Georgia
- pHHyDHODH-GFP Expression Plasmid (for expression in falciparum) from Drexel University
- Thiamethoxam Antibodies from the United States Department of Agriculture/USDA


