The big world of nanotechnology
Nanotechnology refers to matter between 1 and 100 nanometers, known as the nanoscale. A nanoparticle is 10-100nm in one dimension and is defined to behave as a single, whole entity. Nanoparticles are used in a variety of systems, including energy, textiles, cosmetics, sports and medicine. Scientists and engineers have been working with nanoparticles to improve our everyday lives, including to develop items such as stain-resistant clothing, easy-to-clean cookware and lighter running shoes. Nanomedicines are another application of nanotechnology, particularly in drug delivery systems.
 Although modern medicine has positively impacted the lives of many cancer patients by providing longer survival, and in some instances cures, many oncology therapeutics have undesirable side-effects. Chemotherapy, a treatment to target the growth rate of tumors, also affects normal cells. Patients experience nausea, headaches and hair loss, among other symptoms. Drugs that are more specific for disease drivers, aka “targeted therapies”, are designed to be more specific for cancer cells, but can have liabilities for normal cells due to off-target effects of the drugs. Nanotechnology could fill that gap by offering unique drug delivery systems that travel through the body to locate and destroy the tumor while bypassing other organs and tissues.
Although modern medicine has positively impacted the lives of many cancer patients by providing longer survival, and in some instances cures, many oncology therapeutics have undesirable side-effects. Chemotherapy, a treatment to target the growth rate of tumors, also affects normal cells. Patients experience nausea, headaches and hair loss, among other symptoms. Drugs that are more specific for disease drivers, aka “targeted therapies”, are designed to be more specific for cancer cells, but can have liabilities for normal cells due to off-target effects of the drugs. Nanotechnology could fill that gap by offering unique drug delivery systems that travel through the body to locate and destroy the tumor while bypassing other organs and tissues.
Enhanced permeability and retention
Because of their size, nanoparticles can cross cell membranes where other drug delivery systems, such as antibodies, cannot. Solid tumors in particular have unusual vasculature and a poor lymphatic drainage system, creating a different interstitial pressure system in the center of the tumor compared to the outside. This feature is called the enhanced permeability and retention effect (EPR). Because of the EPR, nanoparticles are primed to accumulate in the center of the tumor with low clearance from the body. This phenomenon is compared to “naked” small molecule drugs – that is, not attached to a nanoparticle – which are cleared from the body rapidly via the kidneys. Scientists have taken advantage of the EPR and loaded drugs onto nanoparticles for delivery straight to the middle of the tumor. Studies have demonstrated that drugs loaded onto a nanoparticle can have 10 times the retention time at their intended target compared to their unloaded counterparts. The pharmaceutical implications for these studies are significant: patients would be required to take less drug to achieve efficacious exposure levels while decreasing the risk of unwanted side effects.
Drug delivery methods
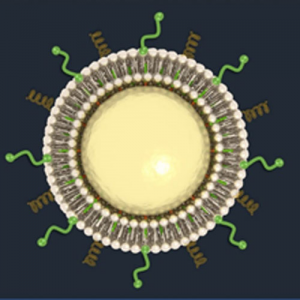
Engineers have piloted several different nanoparticle materials for drug delivery. One popular approach is using liposomes, circular phospholipids made of the same material as cell membranes. Liposomes are used in the delivery of genetic material to cells in vitro, such as treating cells with siRNA, but have also been used for the delivery of anti-cancer agents. When used in this setting, they are termed “nanosomes”. Benefits of nanosomes include their stability and ability to evade the immune system. Drugs that are water-soluble are loaded into the center, while fat-soluble drugs migrate to the phospholipid bilayer itself. In this way, liposomes do not discriminate between molecules based on their physiochemical properties. Furthermore, because they are soluble in blood, nanosomes can boost the efficacy of drugs that were previously limited by their poor solubility through the gut and circulatory system. Several liposome drug delivery systems are approved by the FDA.
Colloidal gold is another common drug delivery system for oncology agents. Colloidal gold is a suspension of gold nanoparticles in a liquid, usually water. Gold nanoparticles have unique physiochemical properties including the ability to bind specific functional groups, such as thiols, that can be linked onto existing drugs. After linking drug to gold and administering to a patient, the nanoparticles migrate to the tumor, taking advantage of the EPR to deliver a potentially fatal strike to the cancer cells. This strategy has been especially useful for broadly toxic agents, such as Doxorubicin, which have posed dosing limitations. Bypassing the normal cells of the body increases the efficacy of these agents and provides patients with much needed benefit.
The big world of nanoparticles has implications stretching across many industries, including medicine. Recent advances in drug delivery systems can potentially overcome many of the drawbacks to already approved anti-cancer therapies. Interested in exploring nanoparticles for your research? Kerafast now offers functionalized gold nanoparticles from the laboratory of Marilyn R. Mackiewicz, PhD at Portland State University. These nanoparticles can be used for live cell imaging and drug delivery exploration. Other nanoparticles available via Kerafast include:
- GlowDot Protein-Based Fluorescent Nanoparticles from the University of Connecticut
- CAN-Fe2O3 Nanoparticles from Bar-Ilan University
References
- Ranganathan, R., Madanmohan, S., Kesavan, A., Baskar, G., Krishnamoorthy, Y. R., Santosham, R., … Venkatraman, G. (2012). Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. International Journal of Nanomedicine, 7, 1043–1060.
- Jain, S., Hirst, D. G., & O’Sullivan, J. M. (2012). Gold nanoparticles as novel agents for cancer therapy. The British Journal of Radiology, 85(1010), 101–113.
- Puri, A., Loomis, K., Smith, B., Lee, J.-H., Yavlovich, A., Heldman, E., & Blumenthal, R. (2009). Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Critical Reviews in Therapeutic Drug Carrier Systems, 26(6), 523–580.


