5 Ways to Improve Your Western Blot
Western blot is a common lab technique used to detect specific proteins in a cell or tissue lysate via antibodies. A misunderstood nemesis, Western blots are a source of headaches and anxieties around the lab. Unlike other protocols, there is no one size fits all approach to blotting – what works for one protein can be disastrous for another. However, to defeat your enemy, you must know your enemy. Follow these five steps to better your Western.
- Understand the difference between gradient and single concentration gels
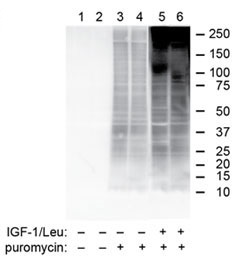
Example Western Blot analysis data, of our puromycin antibody from Penn State College of Medicine
Gels are made of polyacrylamide: a chemically inert, porous material that allows proteins to migrate through when a charge is applied. The size of the pores dictates how proteins of various molecular weights will move through the gel. Most gels are made with either a gradient or one continuous concentration of pore size. When selecting your gel percentage, follow this rule: use a gradient gel to separate proteins of a broad range of sizes; use a single percentage gel to separate proteins that have similar molecular weight. Usually the gel vendor will supply a migration chart to aid in gel selection, but if you are working with new samples, start with a broad gradient, such as 4-20%. After you get to know your samples, move to a tighter gradient or single percentage gel. Using the optimal percentage will make your bands look crisp and publication ready!
- Test a broad range of lysate concentrations
Achieving a clear Western blot depends largely on the abundance of the protein(s) in your sample. If there is not much of your target around, there is a good chance it won’t show up on a gel. If sample is not limiting, doing a titration of your lysate on a gel will give you a general sense of the concentration required to see the target protein. A reasonable titration range is 1-50ug. Still can’t see your protein? It could be an antibody problem (see section 4), or it’s possible that protein is absent or too lowly expressed in your sample.
- Sample blocking buffers
An often-overlooked step in Western blotting, your choice of blocking buffer can make a huge difference in the quality of your gel. Blocking buffers bind to the membrane surface to prevent non-specific interactions of primary antibodies. Blocking agents come in a variety of flavors (i.e. milk, bovine serum albumin, purified protein), and empirical testing will best determine which will be optimal for your specific antibody-protein interaction. For example, milk-containing blocking buffers generally work well for regular proteins, but if you are interested in a phosphorylated protein, a BSA-containing blocking buffer will serve you better. Milk contains a lot of casein, which is a phospho protein itself, so a phospho-specific antibody will pick up the casein and result in high background. Blocking buffers often come in less expensive trial sizes, so you can test to find the optimal method without breaking the bank.
- Choose a primary antibody wisely
Antibody selection is arguably the most important choice in the Western blotting process. Ideally, an antibody should specifically detect its antigen without picking up non-specific proteins or interacting with proteins that are closely related to the target. Some antibody vendors provide notes on the specificity, product reviews and number of publications in which the product has been used. (Here at Kerafast, we provide data, references and comments from customers and the academic investigators who originally created the antibodies in our catalog.) It is worth reading through these sections and comparing the information from different companies. Trying 3-5 antibodies for a protein will usually identify one that clearly detects the target.
- Select a detection method
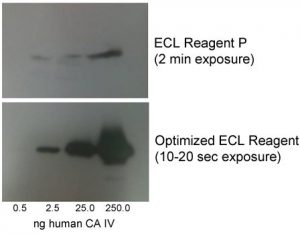
Optimized ECL Reagent, developed by investigators at St. Louis University
The moment you have been waiting for: detection. Today, there are many methods available – X-ray, chemiluminescence, infrared. While each method has its merits and liabilities, the newest and most flexible method is infrared detection. Infrared is more sensitive than traditional systems, which can be useful if your protein of interest is lowly expressed. It also allows for multiplexing, which reduces the number of gels and samples to run. Lastly, because this technology does not rely on an enzymatic reaction, it is thought to be more quantitative and reliable between users. If only X-ray or chemiluminescence is available in your lab, be sure to remain vigilant about exposure time so that your blots are consistent between runs and your data is robust. You may also be interested in an Optimized ECL Reagent, developed by investigators at St. Louis University to offer increased sensitivity and signal and faster exposure times than other commercially available options.
What techniques do you use to improve your Western blotting results? Share your tips and tricks on Facebook and Twitter!


