How the flu virus hijacks host cell RNA
In a recent study in Nature, scientists demonstrated how the influenza virus hijacks host cell RNA to proliferate within the human body. The research identified a specific mechanism common across all influenza strains, providing a new drug target with the potential to treat any influenza infection. In the paper, the investigators used the Influenza A, Nucleoprotein [HT103] Antibody, which is made available on the Kerafast platform by the laboratory of Dr. Thomas M. Moran at the Icahn School of Medicine at Mount Sinai.
According to the World Health Organization, influenza is estimated to cause 3-5 million severe illnesses and 250,000-500,000 deaths each year. Flu vaccines are developed annually to protect against infection, but they only guard against certain strains; in a typical year, vaccination reduces the risk of catching the flu by only 50-60%. An ideal influenza vaccine or drug would be effective against any strain of the virus.
With this aim in mind, researchers from the European Molecular Biology Laboratory and Institut Pasteur in France took a closer look at the influenza virus’ transcription machinery. The influenza virus’ polymerase is required to make viral proteins, but the polymerase is unable to complete the transcription process without RNA from the host cell.
In the current study, the researchers found that the flu polymerase must bind to human polymerase to obtain the RNA it needs to replicate. Using X-ray crystallography, the team determined exactly how the two polymerases interact. They then altered the viral polymerase in a way that prevented the two polymerases from binding, successfully inhibiting viral replication.
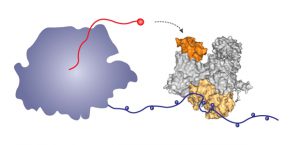
To make human proteins, the human polymerase (left) produces messenger RNA (red). The influenza polymerase (right) binds via its beige region to the long tail of the human polymerase, allowing it to grab hold of the RNA (red) and pirate it to direct production of viral messenger RNA and hence viral proteins. Image credit: Maria Lukaska/EMBL.
The researchers initially worked on a flu strain that only infects bats, but further experimentation showed that blocking polymerase binding also prevents viral proliferation in human cells.
“We’ve uncovered the details of a mechanism that’s common to all influenza strains, so we believe this could be a good target for developing new flu drugs,” said senior author Dr. Stephen Cusack.
Do you work in this area of research? The Kerafast platform offers a variety of virology and immunology reagents direct from academic laboratories worldwide, including influenza antibodies from University of Manitoba and Icahn School of Medicine at Mount Sinai.
For more on influenza research, check out our previous blog post covering a National Institute of Allergy and Infectious Diseases (NIAID) study about a better way to evaluate the effectiveness of flu vaccine candidates.


