Click Chemistry 101
Click chemistry is a versatile reaction used to join two molecules together rapidly and with high yield. Those molecules might be proteins, peptides, DNA, oligonucleotides, small compounds such as drugs, or labeling tools such as biotin and fluorescent dyes. With so many potential applications, click chemistry is a handy tool for many different researchers, ranging from cell biologists to organic chemists. Here you’ll find a quick overview of the process, several example applications, and a look at the reagents making click chemistry possible.
The term “click chemistry” was coined in 1998 by K. Barry Sharpless, who was awarded the 2001 Nobel Prize in Chemistry for his contributions to biorthogonal chemistry. Click chemistry refers to a quick and simple reaction, often catalyzed by copper, which brings together an alkyne (molecule containing a carbon-carbon triple bond) and an azide (an N3 group). The figure below provides a visual outline of the process.
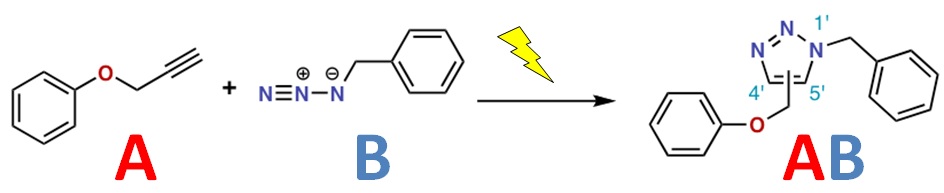
The click chemistry reaction is highly selective, occurring only between azide and alkyne components, both of which are almost never found in natural biomolecules. It therefore doesn’t interfere with native biochemical processes. In addition, the reaction takes place in water over a wide range of pH values, and it can be accomplished by following a simple protocol that doesn’t require a strong chemistry background. Click chemistry has therefore become a widely used tool.
Click chemistry can be used for a variety of applications. Examples include:
1. Creating Fluorescent Antibodies
Click chemistry can create a fluorescent antibody for use in downstream experimentation. First, you would react an azide NHS ester with your antibody of choice. Then, using click chemistry, you would attach the antibody-azide compound to a fluorescent alkyne, thereby generating fluorescent-labeled antibodies that can be used for immunofluorescence (IF) applications.
2. Detecting Sulfenic Acid Formation
Click chemistry can also be used to fluorescently detect sulfenic acid formation in cell culture. For example, you could react a DCP-alkyne with an azide fluorescent dye, such as Cy3-azide. Similarly, you could react DCP-N3 with a fluorescent alkyne. You’d then incubate the new compound with cells and visualize using standard fluorescent detection.
3. Fluorescent Labeling of Proteins, Oligonucleotides or Cells
Click chemistry also offers an effective method for labeling proteins, oligonucleotides and cells. For example, to label an oligonucleotide, you would first introduce an alkyne amidite. Next, the deblocked oligonucleotide would be labeled with a dye azide. The labeled oligonucleotide would then be precipitated with acetone and purified, typically with HPLC.
4. Conjugating Two Proteins
Click chemistry provides a simple way to attach two proteins together. You would react one protein with an alkyne, and the other protein with an azide. The two compounds can then be joined together via click chemistry.
As click chemistry became more popular, researchers began to develop improved molecules for biorthogonal chemistry, so that click chemistry reactions could always occur in the presence of other chemical functionalities found in biological systems without interacting with native biochemical processes. For example, by using a DBCO reagent, the click reaction can occur without the use of a cytotoxic copper catalyst. In addition, the TCO‐tetrazine click reaction offers a powerful tool for catalyst‐free protein-protein bioconjugation, possessing exceptional kinetics and selectivity.
At Kerafast, a variety of click chemistry reagents are available. Vanderbilt University’s Ned A. Porter, PhD and Keri A. Tallman, PhD have contributed biotinylated click chemistry probes, including two that are UV cleavable, as well as alkynated fatty acids, lipid oxidation products and cholesterol derivatives. These compounds’ alkyne moieties allow them to be reacted via copper-catalyzed click chemistry with azide-containing compounds, such as fluorescent or biotin labels.
We also offer a variety of alkynes and azides, as well as catalysts and activators for click chemistry reactions. Our full list of available click chemistry probes can be found here.