MUC1-C: Lubricating for a Cancerous Takeover
Marcos Salazar
March 17, 2014
Mucins consist of a family of glycoproteins that primarily provide protection to epithelial cells exposed to the external environment. They are responsible for forming a gel-like secretion found in the respiratory and gastrointestinal tracts, as well as the linings of ducts, such as those of the mammary glands, liver, pancreas, and kidneys. Mucins are found in two distinct forms: secreted and membrane-bound. While both forms offer protection through the production of a mucous gel, the transmembrane mucins also have the capacity to function as pseudo-cell signaling receptors. They transduce signals during cell stress that promote cell repair through the activation of growth and survival pathways.
Mucin 1 (MUC1) comes in transmembrane form and has been a prominent character in the oncology field. It was first identified in breast cancer cells due to its overexpression pattern. Interestingly, MUC1 is located in chromosome 1q21, a hotspot region with frequent mutations and alterations linked to breast cancer. MUC1 is translated into a single polypeptide, but undergoes autoproteolysis to form a stable heterodimer at the cell membrane. This dimerization has been shown to play an essential role for MUC1 oncogenic activity. The N-terminal subunit, referred to as MUC1-N, is defined by heavy glycosylation and is tethered to the C-terminal subunit, MUC1-C, which is integrated within the plasma membrane.
MUC1 overexpression is found in over 90% of breast tumors. This overexpression is most notably due to genetic mutations and dysregulation of MUC1 transcription. MUC1-C is generally found to accumulate in the cytoplasm of breast cancer cells, leading to constitutive activation of growth and survival pathways. One such pathway that it affects is the PI3K-AKT-mTORC1 pathway. An auto-inductive loop response mechanism is observed, in which MUC1-C activates the PI3K-AKT pathway, and in turn, PI3K-AKT signaling increases MUC1-C translation. MUC1-C also induces RAS signaling through GRB2 and activation of the RAS-MEK-ERK pathway, promoting cell survival. To further complicate matters, MUC1 interacts with and activates various receptor tyrosine kinases, such as epithelial growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), plate-derived growth factor receptor (PDGFR), and ErbB2. Therefore, MUC1-C has its bases covered when it powers up as a cancer-causing player.
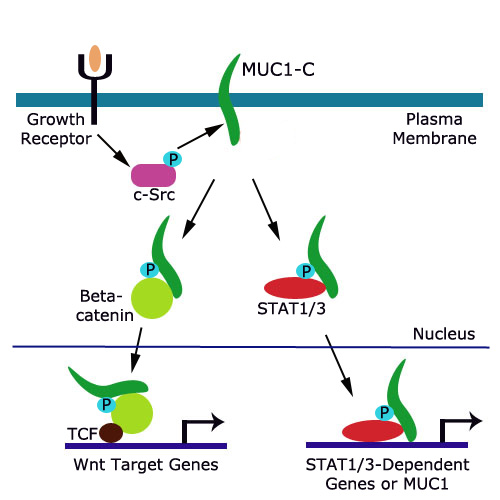
Another way in which MUC1-C leads to a cancerous outcome is with the help of beta-catenin. MUC1-C contains a motif that is specifically recognized by beta-catenin. When this motif is phosphorylated by EGFR or SRC, the affinity for beta-catenin binding is increased. MUC1-C forms a complex with beta-catenin and also interacts with TCF transcription factors to activate Wnt gene transcription. Unregulated activation of this pathway leads to induction of anchorage-independent growth and tumorigenicity.MUC1-C has yet another team member who it calls on to influence tumorigenesis through an inflammatory response mechanism. STAT (signal transducer and activator of transcription) proteins are transcription factors that have been linked to inflammation and cancer. It’s been observed that many breast cancer cell lines possess a prominent STAT1/MUC1-C or STAT3/MUC1-C complex. STATs activate MUC1 expression and lead to an increase in disease recurrence and decrease in overall survival of breast cancer patients. The interaction between STAT1 and MUC1 or STAT3 and MUC1 induces an auto-inductive loop response, much like the MUC1-PI3K-AKT auto-inductive loop, displaying the collaborative nature between inflammation and cancer.
MUC1-C has clinical significance in the oncology field, as patients with primary breast cancer tumors exhibiting MUC1-C overexpression have a significant increase in disease recurrence and morbidity. MUC1-C is a predictive tool of clinical outcome and more importantly, MUC1-C is an attractive drug target. Agents that selectively inhibit MUC1-C may be clinically significant in suppressing or delaying the onset of cancer. Currently, monoclonal antibodies against MUC1-C that target the surface of tumor cells, such as those originating from the breast, are being studied as a potential option for combinational therapy with approved chemotherapy drugs. In addition, small molecules and cell-penetrating peptides are being investigated that inhibit a specific MUC1-C motif necessary for dimerization and oncogenic activity. The targeting of this particular motif leads to suppression of the Wnt and STAT3 pathways in breast cancer cells.
Dr. Donald Kufe, from the Dana-Farber Cancer Institute, has studied the influence of MUC1-C on oncogenesis and, through his work, has generated antibodies that recognize human MUC1-C. For information about the antibodies coming directly from Dr. Kufe’s laboratory, please click on the links below:
Anti-MUC18 (CD146) [OJ79c] Antibody
Tags:
mucins, MUC, MUC1, MUC1-C, MUC1-N, chromosome 1q21, breast cancer, PI3K, AKT, EGFR, FGFR, PDGFR, beta-catenin, STAT1, STAT3, SRC
References:
- Kufe, DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene, 1-9 (2012).
- Kufe, DW. Mucins in cancer: function,prognosis and therapy. Nat Rev Cancer. 9(12):874-85 (2009).