Hitting upon an optimal G Protein alpha purification scheme
Gregory G. Tall, PhD, University of Rochester — Published: April 11, 2013
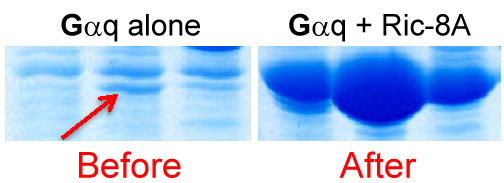
Ric-8A promotes Gαq abundance. Lysates from Sf9 cells expressing Gαq or Gαq and Ric-8A were chromatographed and the Gαq-containing fractions were resolved by SDS-PAGE and Coomassie stained.
Our ability to produce unprecedented quantity of heterotrimeric G protein α subunits was not by design, but brought about by our attempt to solve a technical problem related to a specific aim of a research grant. We had shown that one of two Ric-8 homologs, mammalian Ric-8A was a guanine nucleotide exchange factor (GEF) for 3 out of 4 classes of G protein α subunits (Gαi/q/13). We had good reason to believe that the other Ric-8 homolog, Ric-8B was a GEF for the 4th class, Gαs/olf. At the time, however, we could not purify active Ric-8B protein to test our hypothesis. We attempted to solve this problem by co-purifying Ric-8 proteins with cognate Gα subunits as heterodimeric complexes from Sf9 insect cells. While doing so, we made a dramatic discovery. Not only did we purify the desired Ric-8A:Gα complexes, but we found that the levels of Gα produced in the insect cells were up-regulated dramatically (50-100 fold) by Ric-8 co-expression (See above figure).
We quickly realized that we had a number of advantageous attributes for development of a quality Gα subunit purification scheme: 1. We had an affinity method to trap the complex of GST-Ric-8 and untagged Gα on glutathione resin, 2. We started out with an enhanced abundance of Gα. 3. We knew that we could dissociate the untagged Gα from column-bound GST-Ric-8A because of the GEF properties of Ric-8 (Ric-8 binds nucleotide-free Gα, but not GTP-bound). GTP (mimicry) dissociated the Gα from GST-Ric-8 and allowed Gα to be eluted from the column, while Ric-8A was left behind. The method worked remarkably well and we produced unprecedented amounts of Gα subunits that have been unobtainable by other means.
By all accounts our Gα subunits are fully functional. They appear to be properly lipidated in the eukaryotic Sf9 expression system and they also pass the hallmark test of any functional G protein. When combined with our purified Gβγ subunits, we are able to reconstitute efficient agonist-stimulated G protein GTPγS binding with membrane-expressed G protein coupled receptors (GPCRs).
Tags:
G protein, G protein α subunits, Resistance to Inhibitors of Cholinesterase 8 (Ric-8), protein expression and purification
References:
- Chan P, Gabay M, Wright FA, Kan W, Oner SS, Lanier SM, Smrcka AV, Blumer JB, Tall GG: Purification of Heterotrimeric G Protein α Subunits by GST-Ric-8 Association. Journal of Biological Chemistry 2011, 286:2625-2635.