Puromycin Incorporation as a Measure of Global Protein Synthesis
Andrew R. Kelleher, PhD Candidate, Scot Kimball Lab, Penn State College of Medicine — Published: September 30, 2013
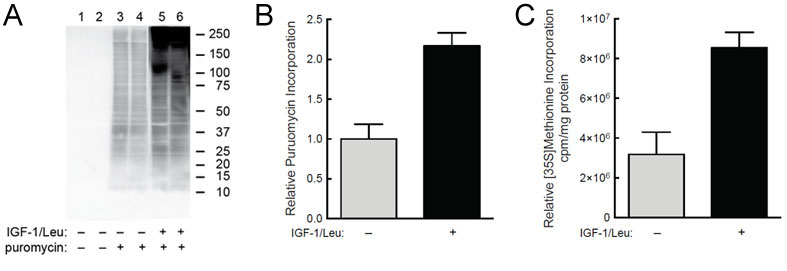
(A) C2C12 myoblasts were starved of serum and leucine for 2 hr and then IGF-1 and leucine were added to the medium of some of the cells for 45 min. Puromycin (1uM) was added to the medium of some of the cells (lanes 3-6) 30 min before harvest. (B) Quantification of western blot analysis from panel A. (C) In the same study, but in a separate set of culture dishes, cells were incubated with [35S]-methionine instead of puromycin and incorporation was measured.
Our laboratory, and my thesis research, is focused on disuse atrophy. Disuse atrophy is the loss of muscle mass with the lack of physical activity. We want to better understand skeletal muscle wasting with disuse and how can we prevent it. We work with an animal model of unilateral hind limb immobilization to simulate disuse atrophy. We are particularly interested in the mechanisms responsible for protein synthesis. In humans, there is a drop in the rate at which proteins are created under conditions of disuse atrophy. So what is the mechanism responsible for this decrease in the rate of skeletal muscle protein synthesis with physical inactivity? To address this question, we need a simple, reliable method of measuring skeletal muscle protein synthesis.
In the past, our lab relied on flooding dose methods using radioactive tracers such as tritiated phenylalanine or [35S]-Methionine as a marker to measure muscle protein synthesis. However, this method was cumbersome and the use of radioactive materials was expensive and dangerous. We were interested in an easier way to measure skeletal muscle protein synthesis. We became familiar with the puromycin molecule which incorporates into newly-synthesized peptides. We then developed a puromycin monoclonal antibody and have been using it ever since.
This antibody opened up all sorts of opportunities for measuring protein synthesis. Now, we simply process the tissue and use quick methods such as western blotting to quantitate overall protein synthesis. This is great because we already routinely used immunoblots of muscle lysates to look at certain protein expression levels or post translational modifications. The antibody also works well for ELISA experiments. This method has been incredible for our lab. The results we obtain with the puromycin antibody are directly comparible to those we obtain using radioactive tracers. We obtain similar increases in protein synthesis as a result of stimuli such as nutrients or leucine and we see similar reductions with disuse.
Learn more about Dr. Kimball’s Anti-Puromycin Monoclonal Antibody »
Tags:
Protein synthesis, translation, puromycin, Anti-Puromycin [3RH11] Antibody.
References:
- Kelleher AR, Kimball, SR, Dennis MD, Schilder RJ and Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab. 304(2):E229-236. 2013.