Engineering an Extended Activity FGF-1
Travis Riedel, PhD, MBA — Published: January 30, 2013
Compared to wild-type, engineered recombinant human FGF-1 variant displays improved overall thermostability, a two-fold increase in resistance to trypsin proteolysis, and a drastic improvement in cell culture half-life (~40 hours, compared to 1 hour for wild-type FGF-1). This unique Extended Activity FGF-1 variant is now available exclusively from The Investigator’s Annexe.
|
Human Fibroblast Growth Factor (FGF-1) is a critical component for maintaining cultured embryonic stem cells in an undifferentiated state. However, FGF is known for its poor thermal stability and degrades over time. As it reaches the end of its life cycle, FGF-1 begins to denature. It is believed that upon denaturation, buried thiol groups within FGF-1 are exposed, leading to aggregation and degradation. Researchers specializing in protein folding and structure at Florida State University designed and produced numerous recombinant FGF-1 variants over the past several years in an attempt to engineer a more stable FGF-1. Based on structural information they collected, the group implemented a mutational strategy that combined the elimination of buried free thiol groups with secondary mutations that enhance overall thermostability. Using isothermal equilibrium denaturation and differential scanning calorimetry, the thermostability of the variants was then analyzed. The most effective FGF-1 variant found consisted of a series of stabilizing point mutations (K12V, C83T, and C117V) and contained a N-terminal polyhistidine-tag. The C83T and C117V mutations resulted in the removal of two of the three total buried free thiol groups, and are proposed to prevent protein aggregation (and subsequent premature degradation) as FGF-1 ends its life cycle. These mutations, however, came at a cost of overall stability compared to wild-type FGF-1. To combat this, a third mutation was introduced at position 12. Lys12 is located at the beginning of the N-terminal β-strand that hydrogen bonds to a β-strand at the C-terminus of the protein, forming an antiparallel β-sheet (see illustration). Substitution of this Lys with Val was shown to further stabilize the protein, presumably through the destabilization of the protein’s denatured state, and compensates for the overall loss of stability due to the Cys mutations. Finally, as part of the purification process, it was observed that the presence of a N-terminal polyhistidine-tag caused marked increase in protein stability. From the FGF-1 structure, it is evident that the His-tag wraps around the protein exterior, providing extra stability (see illustration). The end result was a Human FGF-1 variant which possessed increased resistance to proteolysis, and drastically improved half-life while maintaining WT thermostability. |
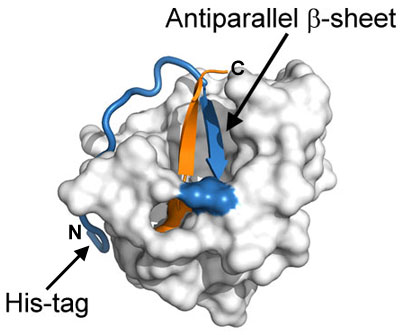
Surface representation of recombinant human FGF-1 (PDB ID: 1JQZ). The critical antiparallel β-sheet is illustrated as a ribbon (N-terminal strand colored in blue, C-terminal strand colored in orange). The N-terminal polyhistidine-tag is shown as a blue cartoon tube. The image above was generated with PyMOL (The PyMOL Molecular Graphics System,Version 1.3, Schrödinger, LLC.). |
Learn more about Extended Activity Recombinant Human FGF-1 »
Tags:
Human Fibroblast Growth Factor, FGF-1, Cell culture, Stem cells, Protein folding, Extended Activity Recombinant Human FGF-1
References:
- Lee J, Blaber M. The Interaction between Thermodynamic Stability and Buried Free Cysteines in Regulating the Functional Half-life of Fibroblast Growth Factor-1, (2009) J. Mol. Biol. 393, 113-127
- Dubey VK, Lee J, Somasundaram T, Blaber S, Blaber M. Spackling the Crack: Stabilizing Human Fibroblast Growth Factor-1 by Targeting ithe N and C terminus Beta-strand Interactions, (2007) J. Mol. Biol. 371, 256-268