Partnering to Advance Research for Tissue Stem Cell-based Biomedicine
James L. Sherley, MD, PhD, Adult Stem Cell Technology Center — Published: January 24, 2014
The Adult Stem Cell Technology Center, LLC (ASCTC) is delighted to partner with KeraFAST to make a unique collection of tissue stem cell strains available to the global research community. Our partnership with KeraFAST paves the way for ASCTC to achieve its mission of enabling advances in tissue stem cell-based biomedicine.
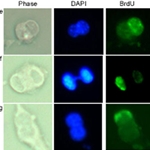
ASCTC provides mouse, rat, and human tissue stem cell strains to undertake research investigations that previously eluded tissue stem cell biologists and cancer cell molecular biologists. Cultures of ASCTC tissue stem cell strains have high fractions of tissue stem cells, ranging from 20% to as high as 70%. This feature makes it possible to perform meaningful investigations of unique tissue stem cell functions and to define genomic and molecular properties of tissue stem cells. Several published reports from ASCTC demonstrate this advantage of the tissue stem cell strains for investigations of tissue stem cell asymmetric self-renewal mechanisms, asymmetric stem cell fate mechanisms, non-random chromosome segregation mechanisms, and discovery of tissue stem cell specific biomarkers across three different mammalian species [1-6].
Another significant advantage of ASCTC’s tissue stem cell strains for research is actually easily overlooked: these strains exhibit normal behavior. The natural purine metabolites used to expand the cell strains are non-mutagenic, and their effect to alter the tissue stem cell self-renewal pattern is entirely reversible. In fact, it is this special quality that provides the ability to conduct molecular investigations of mechanisms that regulate the self-renewal pattern choice (i.e., asymmetric versus symmetric) of tissue stem cells [2,3,5]. In addition, ASCTC reports confirm that these cells are non-tumorigenic in immunodeficient mice [1,6]. Thus, unlike pluripotent stem cells and their differentiated progeny cells, the tissue stem cell strains do not harbor genetic defects that alter their normal function or the normal function of differentiated cells produced from them.
ASCTC tissue stem cell strains also provide important advantages for drug discovery and drug evaluation applications. A vexing problem with pluripotent stem cells is the difficulty inducing them to achieve the differentiated phenotypes of mature post-natal cells instead of earlier stage embryonic and fetal phenotypes. Mature differentiated cell phenotypes are essential for drug development applications. Because ASCTC’s tissue stem cells are derived from post-natal mature tissues, they inherently produce differentiated cells with mature phenotypes [1,3,6].
ASCTC looks forward to a long and productive partnership with KeraFAST. As new tissue stem cell strains become available through expansion of other clinically significant tissues (e.g., pancreatic tissue [6]), we will work with KeraFAST to make them readily available to the global research community.
Learn more about Dr. Sherley’s Adult Stem Cells »
Tags:
Adult stem cells
References:
- Lee, H.-S., Crane, G. G., Merok, J. R., Tunstead, J. R., Hatch, N. L., Panchalingam, K., Powers, M. J., Griffith, L. G., and Sherley, J. L. (2003) “Clonal Expansion of Adult Rat Hepatic Stem Cell Lines by Suppression of Asymmetric Cell Kinetics (SACK)”, Biotech. & Bioeng. 83, 760-771.
- Huh, Y. H. and Sherley, J. L. (2011) “Molecular Cloaking of H2A.Z on Mortal DNA Chromosomes During Non-Random Segregation,” Stem Cells 29, 1620-1627. doi: 10.1002/stem.707.
- Huh, Y. H., King, J., Cohen, J. and Sherley, J. L. (2011) “SACK-Expanded Hair Follicle Stem Cells Display Asymmetric Nuclear Lgr5 Expression with Non-Random Sister Chromatid Segregation,” Sci. Rep. 1, 175; DOI: 10.1038/srep00176.
- Paré, J.-F., and Sherley, J. L. (2011) “Culture Environment-Induced Pluripotency of SACK-Expanded Tissue Stem Cells,” J. Biomed. and Biotechnol. vol. 2011, Article ID 312457, 12 pp., 2011. doi:10.1155/2011/312457.
- Huh, Y. H., Cohen, J., and Sherley, J. L. (2013) “Immortal DNA Strand Chromosomes in Asymmetrically Self-Renewing Distributed Stem Cells Are Marked by Higher 5-Hydroxymethylcytosine Content,” Proc. Natl. Acad. Sci. USA 110, 16862-16867.
- Paré, J.-F., and Sherley, J. L. (2013) “Ex vivo Expansion of Human Pancreatic Distributed Stem Cells by Suppression of Asymmetric Cell Kinetics (SACK),” J. Stem Cell Res. & Therapy 3: 149. doi:10.4172/2157-7633.1000149.